MCED test detects cancer using blood, urine samples
Posted on – Thursday 30 March 23 at 12:45pm

MCED test detects cancer using blood, urine samples
Getting a cancer diagnostic test can be quite traumatic for patients, as not only do they have to prepare mentally and physically for the invasive procedure to submit a tissue sample for biopsy, but there is a time gap between the completion of the test and the arrival of the results. experience stress and anxiety.
Difficult diagnoses are also a barrier to people’s motivation to complete tests on time.
However, over the past few years, independent scientific groups and pharmaceutical companies around the world have been trying to address such issues in cancer diagnosis by developing less invasive, non-invasive, rapid and accurate early cancer detection.
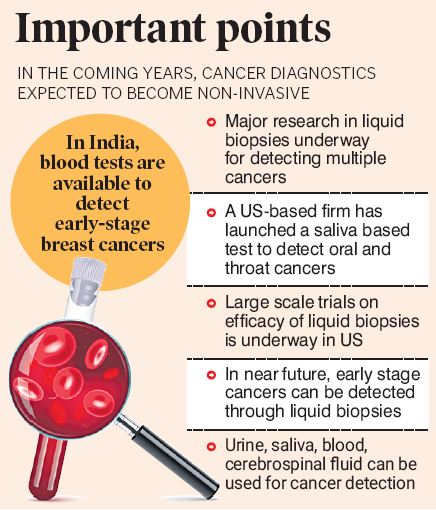
While we are still a few years away from a final non-invasive cancer diagnostic test, cutting-edge research is underway in several countries, most notably the United States, where scientists are developing multiple cancer early detection (MCED) tests using blood or urine samples to detect Various cancers.
Liquid Biopsy or MCED
The National Cancer Institute (NCI), which is involved in developing such tests, defines a liquid biopsy as a laboratory test performed on a sample of blood, urine or other bodily fluids to look for cancer cells in tumors or small masses released by tumor cells into the body DNA, RNA, or other molecules in bodily fluids.
Liquid biopsies allow multiple samples to be taken over time, which may help doctors understand what genetic or molecular changes have occurred in the tumor. Liquid biopsies can be used to help detect cancer early. It can also be used to help make a treatment plan or to see how well treatment is working or if the cancer comes back.
According to the MIT Technology Reviewer, liquid biopsies (blood/urine) work by looking for remnants of tumor cells left after an attack by the immune system. The main rationale behind such tests is that remnants, or fragments, of dead tumors appear in the blood, and collecting and testing these fragments may warn of cancer at an early stage.
Saliva test:
Several pharmaceutical companies are involved in developing simple saliva tests to accurately detect certain cancers. In 2022, the U.S. Food and Drug Administration (USFDA) issued a green signal to launch a saliva-based diagnostic test for oral and throat cancers.
The saliva oral and throat cancer test, developed by US company Viome, is an at-home screening tool based on artificial intelligence and machine learning. The tool analyzes saliva samples for active genes belonging to oral bacteria. When an individual develops a tumor on the lip, tongue or throat, the bacterial population in the mouth changes and the test is able to detect this change.
While traditional cancer diagnostic techniques such as tissue biopsies, mammography, Pap smears, and cervical cancer screening are considered the gold standard, in the next few years doctors may not Requires a scalpel, needle or endoscopy, blood, saliva, cerebrospinal fluid and semen.
